Health
New Surgical Robotic System Set to Transform the Future of Surgery and Patient Care
The University of the Free State (UFS) and the Free State Department of Health mark a transformative moment in healthcare with the launch of the Versius Surgical Robotic System – the first of its kind in Southern Africa.

The University of the Free State (UFS) and the Free State Department of Health are not just introducing new technology, but embarking on a journey that will revolutionize surgery and patient care in the region. This is the message delivered by MaQueen Letsoha-Mathae, Premier of the Free State, during the official launch of the Versius Surgical Robotic System on 11 March 2025 at the UFS Faculty of Health Sciences. The Free State is now the first region in Southern Africa to implement this technology, having successfully completed nine robotic-assisted surgeries at Universitas Academic Hospital within the last month.
The successful procedures, which took place between 24 February and 6 March, included complex surgeries such as radical prostatectomies and cholecystectomies, demonstrating the Versius system’s potential to improve patient outcomes. Prof Freddie Claassen, Academic Head of the Department of Urology at UFS and Universitas Hospital, was among the first surgeons from the university to be trained on the system and use it in operations.
A Significant Milestone
“This moment marks a significant milestone not only for our beloved Free State, but for the entire health-care landscape in Southern Africa. We are not merely launching a new technology; we are embarking on a journey that will transform the future of surgery and patient care in our province and beyond,” said Letsoha-Mathae.
She emphasized that the introduction of the Versius Surgical Robotic System aligns with the Free State’s vision to become a hub of healthcare innovation and excellence in Southern Africa. “With this groundbreaking system, we are not only enhancing surgical precision, but also significantly improving patient outcomes,” Letsoha-Mathae added.
The Versius Surgical Robotic System is known for its versatility and adaptability, seamlessly integrating into any operating room. It can be used in high-specialty procedures, including thoracic, colorectal, general and upper gastrointestinal, hernia, gynaecology, and urology surgeries.
Prof Hester C. Klopper, Vice-Chancellor and Principal of UFS, reflected on the role of the university in shaping the future of healthcare. “This moment is not just a technological milestone, but a symbol of what we can achieve as an institution when we unite academic excellence, visionary leadership, and a commitment to community impact with partners in the private sector and government,” she said.
She stressed that the launch reaffirms UFS’s ongoing dedication to academic excellence, technological innovation, and societal impact, while addressing some of the most pressing healthcare challenges both regionally and globally. “Versius is an investment in the well-being of our communities and an essential step towards bridging the healthcare gap in our region,” said Prof Klopper.
Enhancing Surgical Precision
Prof Vasu Reddy, Deputy Vice-Chancellor of Research and Internationalisation at UFS, explained the significant benefits of robotic-assisted surgeries. “Robots such as the Versius system are tools that enhance the senses and skills of surgeons during delicate operations. Unlike traditional surgery, which requires large incisions, robotic surgery enables doctors to perform operations with smaller cuts, reduced pain, and less scarring,” he said.
Robotic surgery improves surgical outcomes by allowing for greater precision, accuracy, and reduced chances of complications. “The robots do not tire, they do not lose focus, and they can handle repetitive tasks with ease, making the entire process safer for patients,” Prof Reddy continued. He further highlighted the role of AI and robotics in healthcare, emphasizing that human innovation and machine precision together can elevate the standard of care.
According to Premier Letsoha-Mathae, the Versius Surgical Robotic System represents a significant leap towards enhancing healthcare delivery in Southern Africa. “The benefits of Versius are profound: patients will experience quicker recoveries and an earlier return to work, ultimately leading to a healthier, more productive society,” she said.
The system’s modular and scalable design ensures that it can be integrated into operating rooms with minimal infrastructure changes, making it accessible across both private and state healthcare sectors. This adaptability promises to expand access to robotic-assisted surgery, helping to bridge the gap in healthcare delivery.
Through the collaboration between the Department of Health and UFS, the launch of the Versius Surgical Robotic System is a testament to the shared commitment to addressing regional and global healthcare challenges. It also underscores the importance of continuing innovation and academic excellence in advancing healthcare technology and patient care.
Health
Study Unveils Mucus Molecules That Block Salmonella and Prevent Diarrhea
A new MIT-led study reveals how key mucus molecules naturally shield the gut from dangerous bacteria like Salmonella
A new MIT-led study reveals how key mucus molecules naturally shield the gut from dangerous bacteria like Salmonella. The breakthrough opens new pathways for affordable, preventative treatments for travelers and soldiers at risk of infection.
Researchers at MIT have discovered new powerful ways the body guards itself from dangerous bacteria: by deploying mucins, special molecules in mucus that neutralize microbes and stop infection before it starts. The team identified mucins—especially MUC2 and MUC5AC—in the digestive tract that shut down the genetic machinery Salmonella uses to invade cells and cause diarrhea.
“By using and reformatting this motif from the natural innate immune system, we hope to develop strategies to preventing diarrhea before it even starts. This approach could provide a low-cost solution to a major global health challenge that costs billions annually in lost productivity, health care expenses, and human suffering,” said Katharina Ribbeck, the Andrew and Erna Viterbi Professor of Biological Engineering at MIT, in a media statement.
In experiments, exposing Salmonella to the intestinal mucin MUC2 blocked the bacterial proteins that enable infection, turning off the critical regulator HilD. The study also found that a similar mucin from the stomach, MUC5AC, works the same way—and both molecules appear to protect against multiple foodborne germs triggered by similar genetic switches.
“We discovered that these mucins not only create a physical shield but also actively control whether pathogens can turn on genes needed for infection,” Ribbeck explained in the media statement.
Lead authors Kelsey Wheeler and Michaela Gold say synthetic versions of these mucins could soon be added to oral rehydration salts or chewable tablets, providing practical protection for troops, travelers, and people in high-risk areas. According to Wheeler, “Mucin mimics would particularly shine as preventatives, because that’s how the body evolved mucus — as part of this innate immune system to prevent infection,” she said.
Health
First Case of Rare and Deadly Fungus Identified in Sub-Saharan Africa
It is also the first time the rare and deadly fungal infection has been identified in an HIV-positive patient in the region

Doctors at the University of the Free State (UFS) and the National Health Laboratory Service (NHLS) at the Universitas Academic Hospital have confirmed the first recorded case of S. oblongispora mucormycosis in sub-Saharan Africa. It is also the first time the rare and deadly fungal infection has been identified in an HIV-positive patient in the region.
The case involved a 32-year-old man living with HIV who was admitted to Universitas Academic Hospital with severe swelling on the right side of his face. Despite being on antiretroviral therapy and treatment for hypertension, his condition worsened rapidly. Four days after admission, a CT scan and tissue biopsies were conducted. He died three days later, before a diagnosis could be confirmed.
Landmark discovery
Dr. Bonita van der Westhuizen, Senior Lecturer and Pathologist in the UFS Department of Medical Microbiology, described the discovery as a critical turning point for public health in the region.
“This discovery is significant because it highlights the presence of this fungal pathogen in a region where it may have been previously unrecognised or underreported. It now raises awareness about the diversity of fungal infections affecting immunocompromised populations and underscores the need for improved diagnostics, surveillance, and treatment strategies in the region,” she said in a media statement sent to EdPublica.
The case report, co-authored with Drs. Liska Budding and Christie Esterhuysen from the UFS/NHLS and Prof. Samantha Potgieter from the UFS Department of Internal Medicine, was published in Case Reports in Pathology last month.
Rapid and aggressive disease
Mucormycosis, caused by fungi from the order Mucorales, is known for its speed and severity. The infection can invade blood vessels, spread to vital organs, and resist the body’s immune defences.
“Mucorales fungi are known for their fast growth and ability to invade blood vessels. This allows the infection to spread quickly through the body, potentially reaching vital organs,” Dr. Van der Westhuizen explained in the statement. She added that external factors such as traumatic injuries or hospital-acquired infections can worsen the disease’s progression.
While mucormycosis usually strikes patients with underlying conditions like diabetes, cancer, or organ transplants, S. oblongispora has often been linked to infections in otherwise healthy individuals after traumatic inoculation. The lack of local data, however, means its prevalence in African populations remains unclear.
Diagnostic hurdles
In this case, invasive fungal infection was not initially suspected, and the patient did not receive antifungal medication or surgical treatment. The diagnosis was confirmed only after his death, highlighting the broader challenges of detecting fungal diseases in resource-limited settings.
“This is unfortunately the case with mould infections as most readily available diagnostic methods lack sensitivity and these pathogens take long to grow in the laboratory,” Dr. Van der Westhuizen said. “Fungal diagnostics is a specialised field that requires expertise. However, if clinicians are aware of these infections and they have an increased index of suspicion, appropriate therapy can be initiated even before the results are available.”
Experts warn that even with antifungal drugs and surgical removal of infected tissue, the window for treatment is narrow and survival rates remain low.
Building awareness and research
Dr. Van der Westhuizen is continuing her research on invasive mould infections as part of her PhD, focusing on fungal epidemiology and its impact on vulnerable groups such as HIV patients.
Her goal, she said in the media statement, is “to advance understanding and awareness of invasive mould infections, specifically S. oblongispora, in sub-Saharan Africa and among HIV patients. I aim to improve early diagnosis, treatment strategies, and clinical outcomes, as well as to highlight the importance of monitoring fungal infections in immunocompromised populations.”
She hopes the findings will spur more regional collaboration and investment in fungal diagnostics — an often overlooked but increasingly urgent frontier in infectious disease research.
Health
Giant Human Antibody Found to Act Like a Brace Against Bacterial Toxins
This synergistic bracing action gives IgM a unique advantage in neutralizing bacterial toxins that are exposed to mechanical forces inside the body
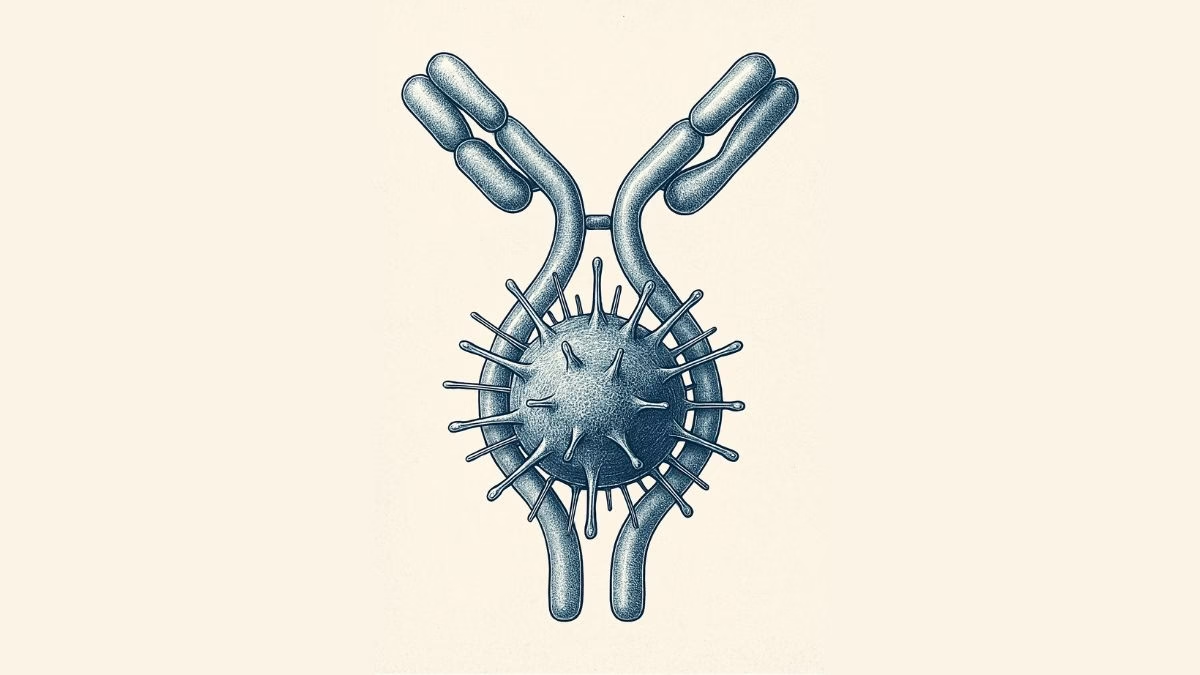
Our immune system’s largest antibody, IgM, has revealed a hidden superpower — it doesn’t just latch onto harmful microbes, it can also act like a brace, mechanically stabilizing bacterial toxins and stopping them from wreaking havoc inside our bodies.
A team of scientists from the S.N. Bose National Centre for Basic Sciences (SNBNCBS) in Kolkata, India, an autonomous institute under the Department of Science and Technology (DST), made this discovery in a recent study. The team reports that IgM can mechanically stiffen bacterial proteins, preventing them from unfolding or losing shape under physical stress.
“This changes the way we think about antibodies,” the researchers said in a media statement. “Traditionally, antibodies are seen as chemical keys that unlock and disable pathogens. But we show they can also serve as mechanical engineers, altering the physical properties of proteins to protect human cells.”
Unlocking a new antibody role
Our immune system produces many different antibodies, each with a distinct function. IgM, the largest and one of the very first antibodies generated when our body detects an infection, has long been recognized for its front-line defense role. But until now, little was known about its ability to physically stabilize dangerous bacterial proteins.
The SNBNCBS study focused on Protein L, a molecule produced by Finegoldia magna. This bacterium is generally harmless but can become pathogenic in certain situations. Protein L acts as a “superantigen,” binding to parts of antibodies in unusual ways and interfering with immune responses.
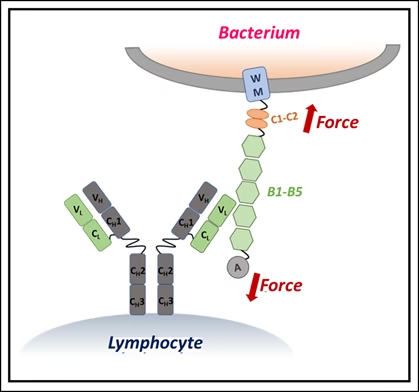
Using single-molecule force spectroscopy — a high-precision method that applies minuscule forces to individual molecules — the researchers discovered that when IgM binds Protein L, the bacterial protein becomes more resistant to mechanical stress. In effect, IgM braces the molecule, preventing it from unfolding under physiological forces, such as those exerted by blood flow or immune cell pressure.
Why size matters
The stabilizing effect depended on IgM concentration: more IgM meant stronger resistance. Simulations showed that this is because IgM’s large structure carries multiple binding sites, allowing it to clamp onto Protein L at several locations simultaneously. Smaller antibodies lack this kind of stabilizing network.
“This synergistic bracing action gives IgM a unique advantage in neutralizing bacterial toxins that are exposed to mechanical forces inside the body,” the researchers explained.
The finding highlights an overlooked dimension of how our immune system works — antibodies don’t merely bind chemically but can also act as mechanical modulators, physically disarming toxins.
Such insights could open a new frontier in drug development, where future therapies may involve engineering antibodies to stiffen harmful proteins, effectively locking them in a harmless state.
The study suggests that by harnessing this natural bracing mechanism, scientists may be able to design innovative treatments that go beyond traditional antibody functions.
-

 Space & Physics5 months ago
Space & Physics5 months agoIs Time Travel Possible? Exploring the Science Behind the Concept
-

 Earth6 months ago
Earth6 months ago122 Forests, 3.2 Million Trees: How One Man Built the World’s Largest Miyawaki Forest
-

 Space & Physics6 months ago
Space & Physics6 months agoDid JWST detect “signs of life” in an alien planet?
-

 Know The Scientist5 months ago
Know The Scientist5 months agoNarlikar – the rare Indian scientist who penned short stories
-

 Society4 months ago
Society4 months agoShukla is now India’s first astronaut in decades to visit outer space
-

 Society4 months ago
Society4 months agoAxiom-4 will see an Indian astronaut depart for outer space after 41 years
-

 Earth4 months ago
Earth4 months agoWorld Environment Day 2025: “Beating plastic pollution”
-

 Society6 months ago
Society6 months agoRabies, Bites, and Policy Gaps: One Woman’s Humane Fight for Kerala’s Stray Dogs