Space & Physics
New Dust Models Shed Light on the End Stages of Sun-like Stars
New Insights into Hydrogen-Deficient Stars: Study of Planetary Nebula IC 2003 Reveals Key Evolutionary Clues
Careful modelling of the thermal and ionization structure of planetary nebulae, based on observations from the Vainu Bappu Telescope in Kavalur, Tamil Nadu, India, has enabled astronomers to deepen their understanding of the formation and evolution of these unusual hydrogen-deficient stars.
Planetary nebulae are shells of gas and dust ejected by stars like our Sun after they exhaust the hydrogen and helium fuel in their cores—a fate our Sun is expected to face in approximately 5 billion years. As the star’s core contracts due to the lack of nuclear fusion, it heats up and emits intense far-ultraviolet radiation. In the past, these stars appeared planet-like when viewed through small telescopes, a resemblance that led to their name.
While most stars in this late phase of their lives produce core remnants surrounded by tiny residual hydrogen envelopes, about 25% of them exhibit a deficiency of hydrogen and are instead rich in helium on their surfaces. Some of these stars also display strong mass loss and emission lines of ionized helium, carbon, and oxygen, characteristics identified as Wolf-Rayet (WR) features.
IC 2003, a rare planetary nebula, features a hydrogen-deficient central remnant star with WR characteristics.
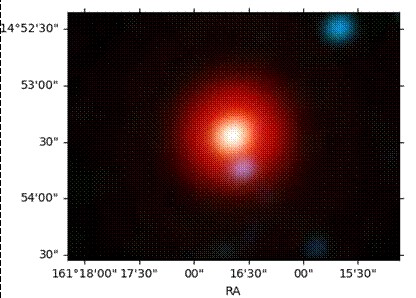
Although the evolutionary status of typical central stars of planetary nebulae is well-understood, the mechanisms behind the formation of hydrogen-poor stars remain largely unclear. The physical and chemical structures of the nebulae surrounding these stars provide valuable clues about their origin and evolution, making it essential to study their gas and dust in detail.
To investigate this further, astronomers from the Indian Institute of Astrophysics (IIA), an autonomous institute under the Department of Science and Technology, observed IC 2003 using the optical medium-resolution spectrograph (OMR) attached to the 2.3-meter Vainu Bappu Telescope at the Vainu Bappu Observatory in Kavalur, Tamil Nadu. “We also used ultraviolet spectra from the IUE satellite and broadband infrared fluxes from the IRAS satellite archives for this study,” said K. Khushbu, the lead author and Ph.D. student. These combined observations provided critical insights into the role of gas and dust in shaping the thermal structure of the nebula, ultimately enabling the team to derive precise parameters for the central star.
The models they used revealed that the nebula’s parameters, including the ionizing source’s mass and temperature, were significantly different from those predicted by dust-free models. “This study highlights the importance of dust grains in the thermal balance of ionized gas and helps explain the large temperature variations seen in nebulae, which are often key to resolving abundance discrepancies in astrophysical nebulae,” explained Prof. C. Muthumariappan, the supervisor and co-author of the study. “We used a one-dimensional dusty photo-ionization code, CLOUDY17.3, to simulate data from ultraviolet, optical, and infrared observations,” he added.
By modeling the photoelectric heating of the nebula caused by the dust grains, the researchers were able to replicate the thermal structure observed in the planetary nebula. “We even reproduced the large temperature gradient typically seen in nebulae with WR stars. Our determination of element abundances, such as helium, nitrogen, and oxygen, differs significantly from the values obtained empirically,” Khushbu noted.
The study also provided accurate grain size distributions within the nebula and highlighted the crucial role of photoelectric heating in explaining temperature variations. From their models, the researchers derived accurate values for the luminosity, temperature, and mass of the central star. They concluded that the star’s initial mass was 3.26 times that of the Sun, indicating it was a more massive star.
This research advances our understanding of the complex processes at work in the evolution of hydrogen-deficient stars and offers valuable insights into the origins of the unique features observed in planetary nebulae.
Space & Physics
Nobel Prize in Physics: Clarke, Devoret, and Martinis Honoured for Pioneering Quantum Discoveries
The 2025 Nobel Prize in Physics honours John Clarke, Michel H. Devoret, and John M. Martinis for revealing how entire electrical circuits can display quantum behaviour — a discovery that paved the way for modern quantum computing.

The 2025 Nobel Prize in Physics has been awarded to John Clarke, Michel H. Devoret, and John M. Martinis for their landmark discovery of macroscopic quantum mechanical tunnelling and energy quantisation in an electric circuit, an innovation that laid the foundation for today’s quantum computing revolution.
Announcing the prize, Olle Eriksson, Chair of the Nobel Committee for Physics, said, “It is wonderful to be able to celebrate the way that century-old quantum mechanics continually offers new surprises. It is also enormously useful, as quantum mechanics is the foundation of all digital technology.”
The Committee described their discovery as a “turning point in understanding how quantum mechanics manifests at the macroscopic scale,” bridging the gap between classical electronics and quantum physics.
John Clarke: The SQUID Pioneer
British-born John Clarke, Professor Emeritus at the University of California, Berkeley, is celebrated for his pioneering work on Superconducting Quantum Interference Devices (SQUIDs) — ultra-sensitive detectors of magnetic flux. His career has been marked by contributions that span superconductivity, quantum amplifiers, and precision measurements.
Clarke’s experiments in the early 1980s provided the first clear evidence of quantum behaviour in electrical circuits — showing that entire electrical systems, not just atoms or photons, can obey the strange laws of quantum mechanics.
A Fellow of the Royal Society, Clarke has been honoured with numerous awards including the Comstock Prize (1999) and the Hughes Medal (2004).
Michel H. Devoret: Architect of Quantum Circuits
French physicist Michel H. Devoret, now the Frederick W. Beinecke Professor Emeritus of Applied Physics at Yale University, has been one of the intellectual architects of quantronics — the study of quantum phenomena in electrical circuits.
After earning his PhD at the University of Paris-Sud and completing a postdoctoral fellowship under Clarke at Berkeley, Devoret helped establish the field of circuit quantum electrodynamics (cQED), which underpins the design of modern superconducting qubits.
His group’s innovations — from the single-electron pump to the fluxonium qubit — have set performance benchmarks in quantum coherence and control. Devoret is also a recipient of the Fritz London Memorial Prize (2014) and the John Stewart Bell Prize, and is a member of the French Academy of Sciences.
John M. Martinis: Building the Quantum Processor
American physicist John M. Martinis, who completed his PhD at UC Berkeley under Clarke’s supervision, translated these quantum principles into the hardware era. His experiments demonstrated energy level quantisation in Josephson junctions, one of the key results now honoured by the Nobel Committee.
Martinis later led Google’s Quantum AI lab, where his team in 2019 achieved the world’s first demonstration of quantum supremacy — showing a superconducting processor outperforming the fastest classical supercomputer on a specific task.
A former professor at UC Santa Barbara, Martinis continues to be a leading voice in quantum computing research and technology development.
A Legacy of Quantum Insight
Together, the trio’s discovery, once seen as a niche curiosity in superconducting circuits, has become the cornerstone of the global quantum revolution. Their experiments proved that macroscopic electrical systems can display quantised energy states and tunnel between them, much like subatomic particles.
Their work, as the Nobel citation puts it, “opened a new window into the quantum behaviour of engineered systems, enabling technologies that are redefining computation, communication, and sensing.”
Space & Physics
The Tiny Grip That Could Reshape Medicine: India’s Dual-Trap Optical Tweezer
Indian scientists build new optical tweezer module—set to transform single-molecule research and medical Innovation
In an inventive leap that could open up new frontiers in neuroscience, drug development, and medical research, scientists in India have designed their own version of a precision laboratory tool known as the dual-trap optical tweezers system. By creating a homegrown solution to manipulate and measure forces on single molecules, the team brings world-class technology within reach of Indian researchers—potentially igniting a wave of scientific discoveries.
Optical tweezers, a Nobel Prize-winning invention from 2018, use focused beams of light to grab and move microscopic objects with extraordinary accuracy. The technique has become indispensable for measuring tiny forces and exploring the mechanics of DNA, proteins, living cells, and engineered nanomaterials. Yet, decades after their invention, conventional optical tweezers systems sometimes fall short for today’s most challenging experiments.
Researchers at the Raman Research Institute (RRI), an autonomous institute backed by India’s Department of Science and Technology in Bengaluru, have now introduced a smart upgrade that addresses long-standing pitfalls of dual-trap tweezers. Traditional setups rely on measuring the light that passes through particles trapped in two separate beams—a method prone to signal “cross-talk.” This makes simultaneous, independent measurement difficult, diminishing both accuracy and versatility.
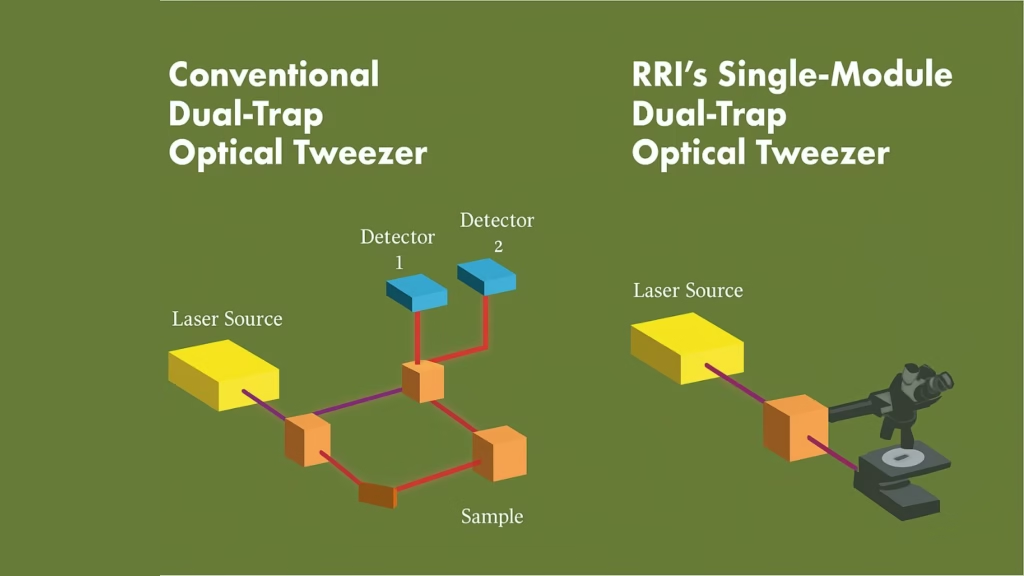
The new system pioneers a confocal detection scheme. In a media statement, Md Arsalan Ashraf, a doctoral scholar at RRI, explained, “The unique optical trapping scheme utilizes laser light scattered back by the sample for detecting trapped particle position. This technique pushes past some of the long-standing constraints of dual-trap configurations and removes signal interference. The single-module design integrates effortlessly with standard microscopy frameworks,” he said.
The refinement doesn’t end there. The system ensures that detectors tracking tiny particles remain perfectly aligned, even when the optical traps themselves move. The result: two stable, reliable measurement channels, zero interference, and no need for complicated re-adjustment mid-experiment—a frequent headache with older systems.
Traditional dual-trap designs have required costly and complex add-ons, sometimes even hijacking the features of laboratory microscopes and making additional techniques, such as phase contrast or fluorescence imaging, hard to use. “This new single-module trapping and detection design makes high-precision force measurement studies of single molecules, probing of soft materials including biological samples, and micromanipulation of biological samples like cells much more convenient and cost-effective,” said Pramod A Pullarkat, lead principal investigator at RRI, in a statement.
By removing cross-talk and offering robust stability—whether traps are close together, displaced, or the environment changes—the RRI team’s approach is not only easier to use but far more adaptable. Its plug-and-play module fits onto standard microscopes without overhauling their basic structure.
From the intellectual property point of view, this design may be a game-changer. By cracking the persistent problem of signal interference with minimalist engineering, the new setup enhances measurement precision and reliability—essential advantages for researchers performing delicate biophysical experiments on everything from molecular motors to living cells.
With the essential building blocks in place, the RRI team is now exploring commercial avenues to produce and distribute their single-module, dual-trap optical tweezer system as an affordable add-on for existing microscopes. The innovation stands to put advanced single-molecule force spectroscopy, long limited to wealthier labs abroad, into the hands of scientists across India—and perhaps spark breakthroughs across the biomedical sciences.
Space & Physics
New Magnetic Transistor Breakthrough May Revolutionize Electronics
A team of MIT physicists has created a magnetic transistor that could make future electronics smaller, faster, and more energy-efficient. By swapping silicon for a new magnetic semiconductor, they’ve opened the door to game-changing advancements in computing.
For decades, silicon has been the undisputed workhorse in transistors—the microscopic switches responsible for processing information in every phone, computer, and high-tech device. But silicon’s physical limits have long frustrated scientists seeking ever-smaller, more efficient electronics.
Now, MIT researchers have unveiled a major advance: they’ve replaced silicon with a magnetic semiconductor, introducing magnetism into transistors in a way that promises tighter, smarter, and more energy-saving circuits. This new ingredient, chromium sulfur bromide, makes it possible to control electricity flow with far greater efficiency and could even allow each transistor to “remember” information, simplifying circuit design for future chips.
“This lack of contamination enables their device to outperform existing magnetic transistors. Most others can only create a weak magnetic effect, changing the flow of current by a few percent or less. Their new transistor can switch or amplify the electric current by a factor of 10,” the MIT team said in a media statement. Their work, detailed in Physical Review Letters, outlines how this material’s stability and clean switching between magnetic states unlocks a new degree of control.
Chung-Tao Chou, MIT graduate student and co-lead author, explains in a media statement, “People have known about magnets for thousands of years, but there are very limited ways to incorporate magnetism into electronics. We have shown a new way to efficiently utilize magnetism that opens up a lot of possibilities for future applications and research.”
The device’s game-changing aspect is its ability to combine the roles of memory cell and transistor, allowing electronics to read and store information faster and more reliably. “Now, not only are transistors turning on and off, they are also remembering information. And because we can switch the transistor with greater magnitude, the signal is much stronger so we can read out the information faster, and in a much more reliable way,” said Luqiao Liu, MIT associate professor, in a media statement.
Moving forward, the team is looking to scale up their clean manufacturing process, hoping to create arrays of these magnetic transistors for broader commercial and scientific use. If successful, the innovation could usher in a new era of spintronic devices, where magnetism becomes as central to electronics as silicon is today.
-

 Space & Physics5 months ago
Space & Physics5 months agoIs Time Travel Possible? Exploring the Science Behind the Concept
-

 Earth6 months ago
Earth6 months ago122 Forests, 3.2 Million Trees: How One Man Built the World’s Largest Miyawaki Forest
-

 Space & Physics6 months ago
Space & Physics6 months agoDid JWST detect “signs of life” in an alien planet?
-

 Know The Scientist5 months ago
Know The Scientist5 months agoNarlikar – the rare Indian scientist who penned short stories
-

 Society4 months ago
Society4 months agoShukla is now India’s first astronaut in decades to visit outer space
-

 Society4 months ago
Society4 months agoAxiom-4 will see an Indian astronaut depart for outer space after 41 years
-

 Earth4 months ago
Earth4 months agoWorld Environment Day 2025: “Beating plastic pollution”
-

 Society6 months ago
Society6 months agoRabies, Bites, and Policy Gaps: One Woman’s Humane Fight for Kerala’s Stray Dogs