Space & Physics
What brought carbon to Earth
This marks the first time a complex form of carbon essential for life on Earth has been observed outside the solar system. To learn more about the significance of this discovery, EdPublica interviewed the researchers behind the study– Gabi Wenzel, Ilsa Cooke, and Brett McGuire, who shared their insights on the implications of pyrene’s presence in space and its potential impact on our understanding of star and planet formation
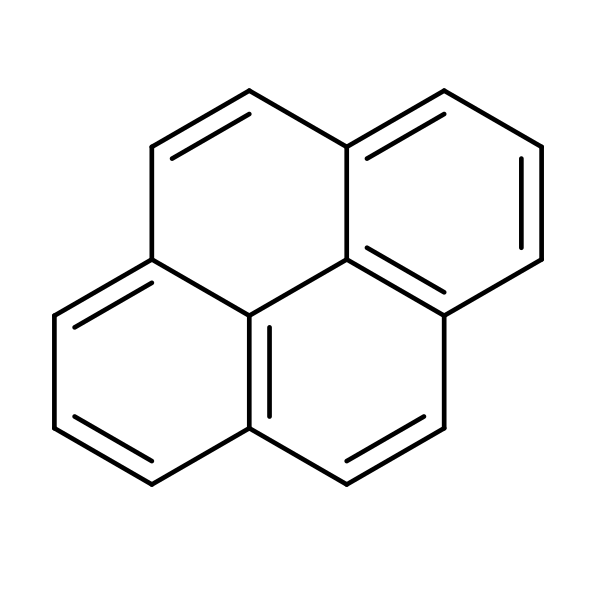
A team led by researchers at MIT has detected pyrene, a complex carbon-containing molecule, in a distant interstellar cloud. This finding opens new avenues for understanding the chemical origins of our solar system. Pyrene, a type of polycyclic aromatic hydrocarbon (PAH), was found in a molecular cloud similar to the one from which our solar system formed.
This marks the first time a complex form of carbon essential for life on Earth has been observed outside the solar system. Its discovery sheds light on how the compounds necessary for life could originate in space. The team detected pyrene in
a star-forming region known as the Taurus Molecular Cloud, located 430 light-years away, making it one of the closest such clouds to Earth.
This discovery also aligns with recent findings from the asteroid Ryugu, suggesting that pyrene may have played a key role in the carbon composition of the early solar system. To learn more about the significance of this discovery, EdPublica interviewed the researchers behind the study– Gabi Wenzel, Ilsa Cooke, and Brett McGuire, who shared their insights on the implications of pyrene’s presence in space and its potential impact on our understanding of star and planet formation. Brett McGuire is an assistant professor of chemistry at MIT, Ilsa Cooke is an assistant professor of chemistry at the University of British Columbia, and Gabi Wenzel is a postdoctoral researcher in McGuire’s group at MIT.
Below, the team responds to questions from EdPublica Editor Dipin Damodharan about this unexpected and exciting discovery.
‘Pyrene could be a major source of carbon in our solar system’
Q: How does the discovery of pyrene in TMC-1 enhance our understanding of the chemical inventory that contributed to the formation of our solar system?
Gabi Wenzel:
Stars much like our own sun are born from dense molecular clouds. The discovery of pyrene in a molecular cloud called TMC-1, one that might be very similar to our sun’s natal cloud and which will go on to form a star of its own, significantly enhances our understanding of the chemical inventory that contributed to the formation of our own solar system. As a polycyclic aromatic hydrocarbon (PAH), pyrene is one of the most complex organic molecules found in early molecular clouds, suggesting that the building blocks of organic matter were available in the environments where stars and their orbiting (exo)planets form.

This discovery sheds light on the chemical processes occurring in interstellar space, including gas-phase and surface reactions on dust grains, which are crucial for the evolution of organic chemistry. This further supports the notion that the primordial materials of our solar system contained a diverse range of organic compounds, providing insights into the potential for prebiotic chemistry on a young Earth and planetesimals.
Q: What specific challenges did you face in detecting pyrene, given that it is invisible to traditional radio astronomy methods, and how did the use of cyanopyrene help overcome these challenges?
Gabi Wenzel:
Pyrene, a fully symmetric PAH, does not possess a permanent electric dipole moment and hence is invisible in radio astronomical observations or rotational spectrometers in the laboratory. The CN radical is highly abundant in the cold and dark molecular cloud TMC-1, an environment that is about 10 K cold and in which you’d assume little chemistry to happen. However, earlier experimental works have shown that the CN addition (followed by hydrogen abstraction) to ringed hydrocarbon species such as benzene and toluene at low temperatures is a barrierless process.
Adding a CN (nitrile) group to a hydrocarbon will drastically increase its permanent electric dipole moment and so allow rotational transitions. Indeed, several CN-functionalized species have been detected in TMC-1 and other sources, among which the CN-substituted benzene (cyanobenzene or benzonitrile) and other smaller PAHs, with cyanopyrene being the largest molecule found via radio astronomy to date, allowing us to infer the presence of pyrene itself.
Q: Can you elaborate on what it means for our understanding of carbon sources in the solar system that pyrene is found in both TMC-1 and asteroid Ryugu?
Ilsa Cooke:
TMC-1 is a famous example of a cold molecular cloud, one of the earliest stages of star and planet formation, while asteroids like Ryugu represent snapshots of later stages in the formation of solar systems. Asteroids are formed from material in the solar nebula that was inherited from the molecular cloud stage. Our radio observations of TMC-1 let us observe pyrene early on and possibly under conditions where it is first forming. Isotope signatures of the pyrene in Ryugu suggest it was formed in a cold interstellar cloud. From these two unique sets of measurements, we can start to unravel the inheritance of pyrene, and PAHs more generally, from their birth in interstellar space and their journey to new planets. If PAHs can survive all the way from the molecular cloud stage, they may provide planets with an important source of organic carbon.

Q: What are the different formation routes of PAHs that your research suggests, and how do these differ from previous hypotheses about PAH formation in space?
Ilsa Cooke:
Our results, combined with those of Zeichner et al., who measured pyrene in Ryugu, suggest that pyrene may form at low temperatures by “bottom-up” routes in molecular clouds. Previously, PAHs were most commonly associated with formation in high-temperature (ca. 1000 K) environments in the envelopes of dying stars. These stars are thought to eject their PAHs, along with other carbon-rich molecules, into the diffuse interstellar medium.

However, the diffuse medium is a tenuous, harsh environment permeated by ultraviolet photons, and most astrochemists think that small PAHs would not survive their journey through the diffuse medium into dense molecular clouds. So we are still left with a puzzle: does that pyrene that we observe in TMC-1 form there, or was it formed somewhere else but it was able to survive its journey more efficiently than previously thought? If the pyrene is indeed formed within TMC-1, we do not yet know the chemical mechanism. Many possibilities exist, so close collaborations between laboratory astrochemists and observers will be critical to answer this question.
Q: What are your plans for investigating larger PAH molecules in TMC-1, and what specific hypotheses are you looking to test with these investigations?
Brett McGuire:
We have a number of other targets lined up – again focusing on PAH structures that should show this special stability demonstrated by pyrene. They present the same experimental challenges, including needing to devise appropriate synthetic routes in the laboratory before collecting their spectra. The major question is just how complex the PAH inventory actually gets at this earliest stage of star formation.
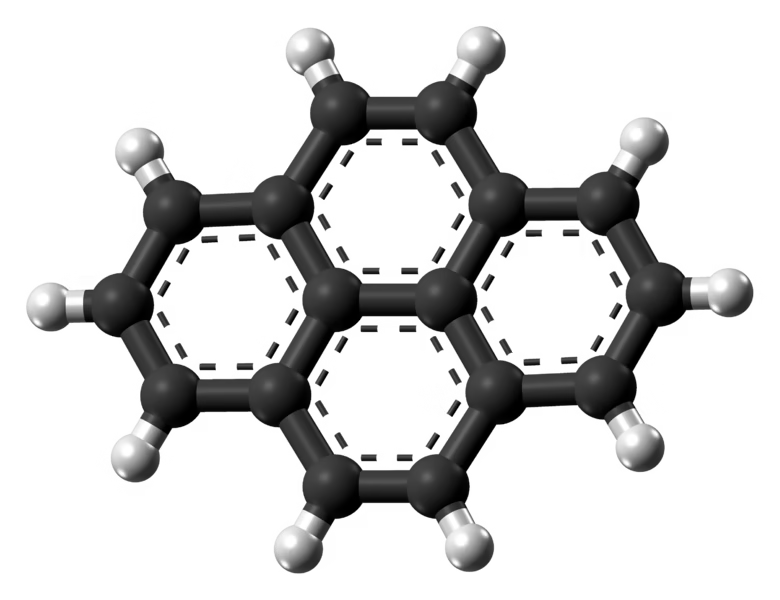
Prior to our work in TMC-1, nearly everything we knew about PAHs came from infrared observations of bulk properties in much warmer and more energetic regions, where PAHs are thought to be much larger. Does the population in TMC-1 look the same as in these regions? Is it at an earlier stage of chemical evolution? And how does this distribution compare to what we see in our own Solar System?

Q: How do your findings about pyrene and PAHs in interstellar clouds influence our broader understanding of organic chemistry in the universe, particularly in relation to the origins of life?
Brett McGuire:
Life as we know it depends on carbon – it is the backbone upon which all our molecular structures are constructed. Yet, the Earth overall is somewhat depleted in carbon relative to what we’d naively expect, and we still don’t fully understand where the carbon we do have came from originally. PAHs in general seem to be a massive reservoir of reactive carbon, and what we are now seeing is that that reservoir is already present at the earliest stages of star-formation. Combined with the evidence from Ryugu, we’re now also seeing indications that the inventory of PAHs, and thus this reservoir of carbon, may actually survive from this dark molecular cloud phase through the formation of a star to be eventually incorporated into the planetary system itself.
Space & Physics
MIT Pioneers Real-Time Observation of Unconventional Superconductivity in Magic-Angle Graphene
Physicists have directly observed unconventional superconductivity in magic-angle twisted tri-layer graphene using a new experimental platform, revealing a unique pairing mechanism
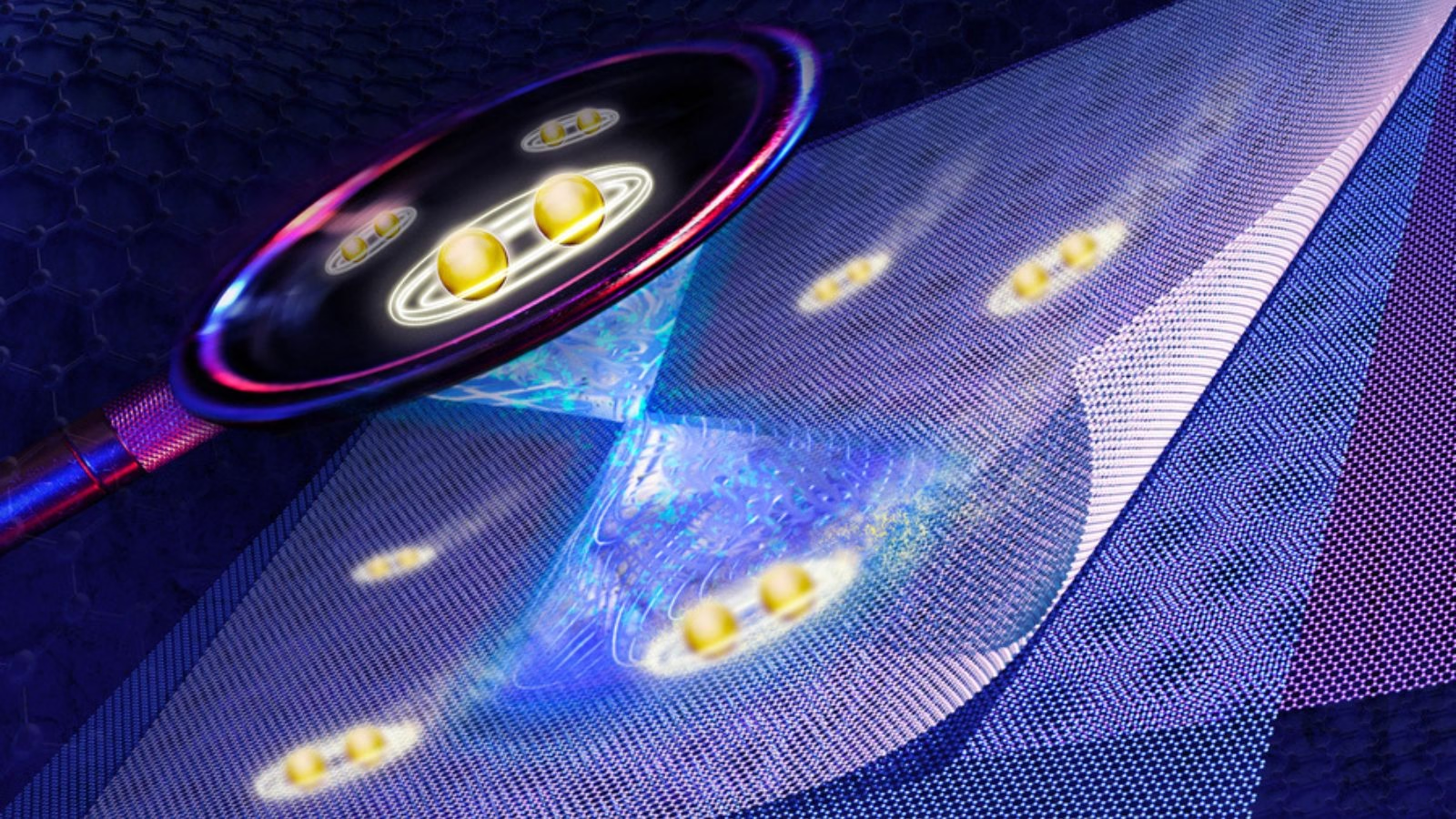
MIT physicists have unveiled compelling direct evidence for unconventional superconductivity in “magic-angle” twisted tri-layer graphene—an atomically engineered material that could reimagine the future of energy transport and quantum technologies. Their new experiment marks a pivotal step forward, offering a fresh perspective on how electrons synchronize in precisely stacked two-dimensional materials, potentially laying the groundwork for next-generation superconductors that function well above current temperature limits.
Instead of looking merely at theoretical possibilities, the MIT team built a novel platform that lets researchers visualize the superconducting gap “as it emerges in real-time within 2D materials,” said co-lead author Shuwen Sun in a media statement. This gap is crucial, reflecting how robust the material’s superconducting state is during temperature changes—a key indicator for practical applications.
What’s striking, said Jeong Min Park, study co-lead author, is that the superconducting gap in magic-angle graphene differs starkly from the smooth, uniform profile seen in conventional superconductors. “We observed a V-shaped gap that reveals an entirely new pairing mechanism—possibly driven by the electrons themselves, rather than crystal vibrations,” Park said. Such direct measurement is a “first” for the field, giving scientists a more refined tool for identifying and understanding unconventional superconductivity.
Senior author Pablo Jarillo-Herrero emphasized that their method could help crack the code behind room-temperature superconductors: “This breakthrough may trigger deeper insights not just for graphene, but for the entire class of twistronic materials. Imagine grids and quantum computers that operate with zero energy loss—this is the holy grail we’re moving toward,” Jarillo-Herrero said in the MIT release.
Collaborators included scientists from Japan’s National Institute for Materials Science, broadening the impact of the research. The discovery builds on years of progress since the first magic-angle graphene experiments in 2018, opening what many now call the “twistronics” era—a field driven by stacking and twisting atom-thin materials to unlock uniquely quantum properties.
Looking ahead, the team plans to expand its analysis to other ultra-thin structures, hoping to map out electronic behavior not only for superconductors, but for a wider range of correlated quantum phases. “We can now directly observe electron pairs compete and coexist with other quantum states—this could allow us to design new materials from the ground up,” said Park in her public statement.
The research underscores the value of visualization in fundamental physics, suggesting that direct observation may be the missing link to controlling quantum phenomena for efficient, room-temperature technology.
Space & Physics
Atoms Speak Out: Physicists Use Electrons as Messengers to Unlock Secrets of the Nucleus
Physicists at MIT have devised a table-top method to peer inside an atom’s nucleus using the atom’s own electrons
Physicists at MIT have developed a pioneering method to look inside an atom’s nucleus — using the atom’s own electrons as tiny messengers within molecules rather than massive particle accelerators.
In a study published in science, the researchers demonstrated this approach using molecules of radium monofluoride, which pair a radioactive radium atom with a fluoride atom. The molecules act like miniature laboratories where electrons naturally experience extremely strong electric fields. Under these conditions, some electrons briefly penetrate the radium nucleus, interacting directly with protons and neutrons inside. This rare intrusion leaves behind a measurable energy shift, allowing scientists to infer details about the nucleus’ internal structure.
The team observed that these energy shifts, though minute — about one millionth of the energy of a laser photon — provide unambiguous evidence of interactions occurring inside the nucleus rather than outside it. “We now have proof that we can sample inside the nucleus,” said Ronald Fernando Garcia Ruiz, the Thomas A. Franck Associate Professor of Physics at MIT, in a statement. “It’s like being able to measure a battery’s electric field. People can measure its field outside, but to measure inside the battery is far more challenging. And that’s what we can do now.”
Traditionally, exploring nuclear interiors required kilometer-long particle accelerators to smash high-speed beams of electrons into targets. The MIT technique, by contrast, achieves similar insight with a table-top molecular setup. It makes use of the molecule’s natural electric environment to magnify these subtle interactions.
The radium nucleus, unlike most which are spherical, has an asymmetric “pear” shape that makes it a powerful system for studying violations of fundamental physical symmetries — phenomena that could help explain why the universe contains far more matter than antimatter. “The radium nucleus is predicted to be an amplifier of this symmetry breaking, because its nucleus is asymmetric in charge and mass, which is quite unusual,” Garcia Ruiz explained.
To conduct their experiments, the researchers produced radium monofluoride molecules at CERN’s Collinear Resonance Ionization Spectroscopy (CRIS) facility, trapped and cooled them in laser-guided chambers, and then measured laser-induced energy transitions with extreme precision. The work involved MIT physicists Shane Wilkins, Silviu-Marian Udrescu, and Alex Brinson, alongside international collaborators.
“Radium is naturally radioactive, with a short lifetime, and we can currently only produce radium monofluoride molecules in tiny quantities,” said Wilkins. “We therefore need incredibly sensitive techniques to be able to measure them.”
As Udrescu added, “When you put this radioactive atom inside of a molecule, the internal electric field that its electrons experience is orders of magnitude larger compared to the fields we can produce and apply in a lab. In a way, the molecule acts like a giant particle collider and gives us a better chance to probe the radium’s nucleus.”
Going forward, the MIT team aims to cool and align these molecules so that the orientation of their pear-shaped nuclei can be controlled for even more detailed mapping. “Radium-containing molecules are predicted to be exceptionally sensitive systems in which to search for violations of the fundamental symmetries of nature,” Garcia Ruiz said. “We now have a way to carry out that search”
Space & Physics
Physicists Double Precision of Optical Atomic Clocks with New Laser Technique
MIT researchers develop a quantum-enhanced method that doubles the precision and stability of optical atomic clocks, paving the way for portable, ultra-accurate timekeeping.
MIT physicists have unveiled a new technique that could significantly improve the precision and stability of next-generation optical atomic clocks, devices that underpin everything from mobile transactions to navigation apps. In a recent media statement, the MIT team explained: “Every time you check the time on your phone, make an online transaction, or use a navigation app, you are depending on the precision of atomic clocks. An atomic clock keeps time by relying on the ‘ticks’ of atoms as they naturally oscillate at rock-steady frequencies.”
Current atomic clocks rely on cesium atoms tracked with lasers at microwave frequencies, but scientists are advancing to clocks based on faster-ticking atoms like ytterbium, which can be tracked with lasers at higher, optical frequencies and discern intervals up to 100 trillion times per second.
A research group at MIT, led by Vladan Vuletić, the Lester Wolfe Professor of Physics, detailed that their newly developed method harnesses a laser-induced “global phase” in ytterbium atoms and boosts this effect using quantum amplification. Vuletić stated, “We think our method can help make these clocks transportable and deployable to where they’re needed.” The approach, called global phase spectroscopy, doubles the precision of an optical atomic clock, enabling it to resolve twice as many ticks per second compared to standard setups, and promises further gains with increasing atom counts.
The technique could pave the way for portable optical atomic clocks able to measure all manner of phenomena in various locations. Vuletić summarized the broader scientific ambitions: “With these clocks, people are trying to detect dark matter and dark energy, and test whether there really are just four fundamental forces, and even to see if these clocks can predict earthquakes.”
The MIT team has previously demonstrated improved clock precision by quantumly entangling hundreds of ytterbium atoms and using time reversal tricks to amplify their signals. Their latest advance applies these methods to much faster optical frequencies, where stabilizing the clock laser has always been a major challenge. “When you have atoms that tick 100 trillion times per second, that’s 10,000 times faster than the frequency of microwaves,” said Vuletić in the statement. Their experiments revealed a surprisingly useful “global phase” information about the laser frequency, previously thought irrelevant, unlocking the potential for even greater accuracy.
The research, led by Vuletić and joined by Leon Zaporski, Qi Liu, Gustavo Velez, Matthew Radzihovsky, Zeyang Li, Simone Colombo, and Edwin Pedrozo-Peñafiel of the MIT-Harvard Center for Ultracold Atoms, was published in Nature. They believe the technical benefits of the new method will make atomic clocks easier to run and enable stable, transportable clocks fit for future scientific exploration, including earthquake prediction, fundamental physics, and global time standards.
-

 Space & Physics6 months ago
Space & Physics6 months agoIs Time Travel Possible? Exploring the Science Behind the Concept
-

 Know The Scientist6 months ago
Know The Scientist6 months agoNarlikar – the rare Indian scientist who penned short stories
-

 Know The Scientist5 months ago
Know The Scientist5 months agoRemembering S.N. Bose, the underrated maestro in quantum physics
-

 Space & Physics3 months ago
Space & Physics3 months agoJoint NASA-ISRO radar satellite is the most powerful built to date
-

 Society5 months ago
Society5 months agoAxiom-4 will see an Indian astronaut depart for outer space after 41 years
-

 Society5 months ago
Society5 months agoShukla is now India’s first astronaut in decades to visit outer space
-

 Society5 months ago
Society5 months agoWhy the Arts Matter As Much As Science or Math
-

 Earth5 months ago
Earth5 months agoWorld Environment Day 2025: “Beating plastic pollution”