Space & Physics
What brought carbon to Earth
This marks the first time a complex form of carbon essential for life on Earth has been observed outside the solar system. To learn more about the significance of this discovery, EdPublica interviewed the researchers behind the study– Gabi Wenzel, Ilsa Cooke, and Brett McGuire, who shared their insights on the implications of pyrene’s presence in space and its potential impact on our understanding of star and planet formation
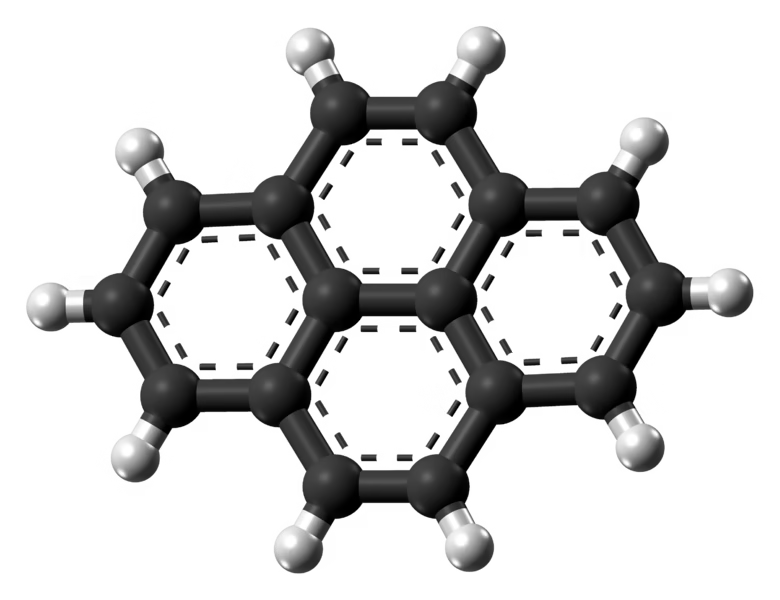
A team led by researchers at MIT has detected pyrene, a complex carbon-containing molecule, in a distant interstellar cloud. This finding opens new avenues for understanding the chemical origins of our solar system. Pyrene, a type of polycyclic aromatic hydrocarbon (PAH), was found in a molecular cloud similar to the one from which our solar system formed.
This marks the first time a complex form of carbon essential for life on Earth has been observed outside the solar system. Its discovery sheds light on how the compounds necessary for life could originate in space. The team detected pyrene in
a star-forming region known as the Taurus Molecular Cloud, located 430 light-years away, making it one of the closest such clouds to Earth.
This discovery also aligns with recent findings from the asteroid Ryugu, suggesting that pyrene may have played a key role in the carbon composition of the early solar system. To learn more about the significance of this discovery, EdPublica interviewed the researchers behind the study– Gabi Wenzel, Ilsa Cooke, and Brett McGuire, who shared their insights on the implications of pyrene’s presence in space and its potential impact on our understanding of star and planet formation. Brett McGuire is an assistant professor of chemistry at MIT, Ilsa Cooke is an assistant professor of chemistry at the University of British Columbia, and Gabi Wenzel is a postdoctoral researcher in McGuire’s group at MIT.
Below, the team responds to questions from EdPublica Editor Dipin Damodharan about this unexpected and exciting discovery.
‘Pyrene could be a major source of carbon in our solar system’
Q: How does the discovery of pyrene in TMC-1 enhance our understanding of the chemical inventory that contributed to the formation of our solar system?
Gabi Wenzel:
Stars much like our own sun are born from dense molecular clouds. The discovery of pyrene in a molecular cloud called TMC-1, one that might be very similar to our sun’s natal cloud and which will go on to form a star of its own, significantly enhances our understanding of the chemical inventory that contributed to the formation of our own solar system. As a polycyclic aromatic hydrocarbon (PAH), pyrene is one of the most complex organic molecules found in early molecular clouds, suggesting that the building blocks of organic matter were available in the environments where stars and their orbiting (exo)planets form.

This discovery sheds light on the chemical processes occurring in interstellar space, including gas-phase and surface reactions on dust grains, which are crucial for the evolution of organic chemistry. This further supports the notion that the primordial materials of our solar system contained a diverse range of organic compounds, providing insights into the potential for prebiotic chemistry on a young Earth and planetesimals.
Q: What specific challenges did you face in detecting pyrene, given that it is invisible to traditional radio astronomy methods, and how did the use of cyanopyrene help overcome these challenges?
Gabi Wenzel:
Pyrene, a fully symmetric PAH, does not possess a permanent electric dipole moment and hence is invisible in radio astronomical observations or rotational spectrometers in the laboratory. The CN radical is highly abundant in the cold and dark molecular cloud TMC-1, an environment that is about 10 K cold and in which you’d assume little chemistry to happen. However, earlier experimental works have shown that the CN addition (followed by hydrogen abstraction) to ringed hydrocarbon species such as benzene and toluene at low temperatures is a barrierless process.
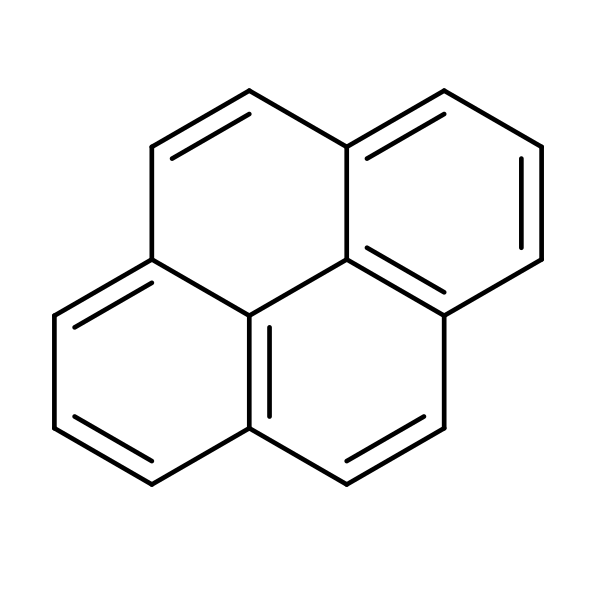
Adding a CN (nitrile) group to a hydrocarbon will drastically increase its permanent electric dipole moment and so allow rotational transitions. Indeed, several CN-functionalized species have been detected in TMC-1 and other sources, among which the CN-substituted benzene (cyanobenzene or benzonitrile) and other smaller PAHs, with cyanopyrene being the largest molecule found via radio astronomy to date, allowing us to infer the presence of pyrene itself.
Q: Can you elaborate on what it means for our understanding of carbon sources in the solar system that pyrene is found in both TMC-1 and asteroid Ryugu?
Ilsa Cooke:
TMC-1 is a famous example of a cold molecular cloud, one of the earliest stages of star and planet formation, while asteroids like Ryugu represent snapshots of later stages in the formation of solar systems. Asteroids are formed from material in the solar nebula that was inherited from the molecular cloud stage. Our radio observations of TMC-1 let us observe pyrene early on and possibly under conditions where it is first forming. Isotope signatures of the pyrene in Ryugu suggest it was formed in a cold interstellar cloud. From these two unique sets of measurements, we can start to unravel the inheritance of pyrene, and PAHs more generally, from their birth in interstellar space and their journey to new planets. If PAHs can survive all the way from the molecular cloud stage, they may provide planets with an important source of organic carbon.

Q: What are the different formation routes of PAHs that your research suggests, and how do these differ from previous hypotheses about PAH formation in space?
Ilsa Cooke:
Our results, combined with those of Zeichner et al., who measured pyrene in Ryugu, suggest that pyrene may form at low temperatures by “bottom-up” routes in molecular clouds. Previously, PAHs were most commonly associated with formation in high-temperature (ca. 1000 K) environments in the envelopes of dying stars. These stars are thought to eject their PAHs, along with other carbon-rich molecules, into the diffuse interstellar medium.

However, the diffuse medium is a tenuous, harsh environment permeated by ultraviolet photons, and most astrochemists think that small PAHs would not survive their journey through the diffuse medium into dense molecular clouds. So we are still left with a puzzle: does that pyrene that we observe in TMC-1 form there, or was it formed somewhere else but it was able to survive its journey more efficiently than previously thought? If the pyrene is indeed formed within TMC-1, we do not yet know the chemical mechanism. Many possibilities exist, so close collaborations between laboratory astrochemists and observers will be critical to answer this question.
Q: What are your plans for investigating larger PAH molecules in TMC-1, and what specific hypotheses are you looking to test with these investigations?
Brett McGuire:
We have a number of other targets lined up – again focusing on PAH structures that should show this special stability demonstrated by pyrene. They present the same experimental challenges, including needing to devise appropriate synthetic routes in the laboratory before collecting their spectra. The major question is just how complex the PAH inventory actually gets at this earliest stage of star formation.
Prior to our work in TMC-1, nearly everything we knew about PAHs came from infrared observations of bulk properties in much warmer and more energetic regions, where PAHs are thought to be much larger. Does the population in TMC-1 look the same as in these regions? Is it at an earlier stage of chemical evolution? And how does this distribution compare to what we see in our own Solar System?

Q: How do your findings about pyrene and PAHs in interstellar clouds influence our broader understanding of organic chemistry in the universe, particularly in relation to the origins of life?
Brett McGuire:
Life as we know it depends on carbon – it is the backbone upon which all our molecular structures are constructed. Yet, the Earth overall is somewhat depleted in carbon relative to what we’d naively expect, and we still don’t fully understand where the carbon we do have came from originally. PAHs in general seem to be a massive reservoir of reactive carbon, and what we are now seeing is that that reservoir is already present at the earliest stages of star-formation. Combined with the evidence from Ryugu, we’re now also seeing indications that the inventory of PAHs, and thus this reservoir of carbon, may actually survive from this dark molecular cloud phase through the formation of a star to be eventually incorporated into the planetary system itself.
Space & Physics
Physicists Capture ‘Wakes’ Left by Quarks in the Universe’s First Liquid
Scientists at CERN’s Large Hadron Collider have observed, for the first time, fluid-like wakes created by quarks moving through quark–gluon plasma, offering direct evidence that the universe’s earliest matter behaved like a liquid rather than a cloud of free particles.
Physicists working at the CERN(The European Organization for Nuclear Research) have reported the first direct experimental evidence that quark–gluon plasma—the primordial matter that filled the universe moments after the Big Bang—behaves like a true liquid.
Using heavy-ion collisions at the Large Hadron Collider, researchers recreated the extreme conditions of the early universe and observed that quarks moving through this plasma generate wake-like patterns, similar to ripples trailing a duck across water.
The study, led by physicists from the Massachusetts Institute of Technology, shows that the quark–gluon plasma responds collectively, flowing and splashing rather than scattering randomly.
“It has been a long debate in our field, on whether the plasma should respond to a quark,” said Yen-Jie Lee in a media statement. “Now we see the plasma is incredibly dense, such that it is able to slow down a quark, and produces splashes and swirls like a liquid. So quark-gluon plasma really is a primordial soup.”
Quark–gluon plasma is believed to be the first liquid to have existed in the universe and the hottest ever observed, reaching temperatures of several trillion degrees Celsius. It is also considered a near-perfect liquid, flowing with almost no resistance.
To isolate the wake produced by a single quark, the team developed a new experimental technique. Instead of tracking pairs of quarks and antiquarks—whose effects can overlap—they identified rare collision events that produced a single quark traveling in the opposite direction of a Z boson. Because a Z boson interacts weakly with its surroundings, it acts as a clean marker, allowing scientists to attribute any observed plasma ripples solely to the quark.
“We have figured out a new technique that allows us to see the effects of a single quark in the QGP, through a different pair of particles,” Lee said.
Analysing data from around 13 billion heavy-ion collisions, the researchers identified roughly 2,000 Z-boson events. In these cases, they consistently observed fluid-like swirls in the plasma opposite to the Z boson’s direction—clear signatures of quark-induced wakes.
The results align with theoretical predictions made by MIT physicist Krishna Rajagopal, whose hybrid model suggested that quarks should drag plasma along as they move through it.
“This is something that many of us have argued must be there for a good many years, and that many experiments have looked for,” Rajagopal said.
“We’ve gained the first direct evidence that the quark indeed drags more plasma with it as it travels,” Lee added. “This will enable us to study the properties and behavior of this exotic fluid in unprecedented detail.”
The research was carried out by members of the CMS Collaboration using the Compact Muon Solenoid detector at CERN. The open-access study has been published in the journal Physics Letters B.
Space & Physics
Why Jupiter Has Eight Polar Storms — and Saturn Only One: MIT Study Offers New Clues
Two giant planets, made of the same elements, display radically different storms at their poles. New research from MIT now suggests that the key to this cosmic mystery lies not in the skies, but deep inside Jupiter and Saturn themselves.
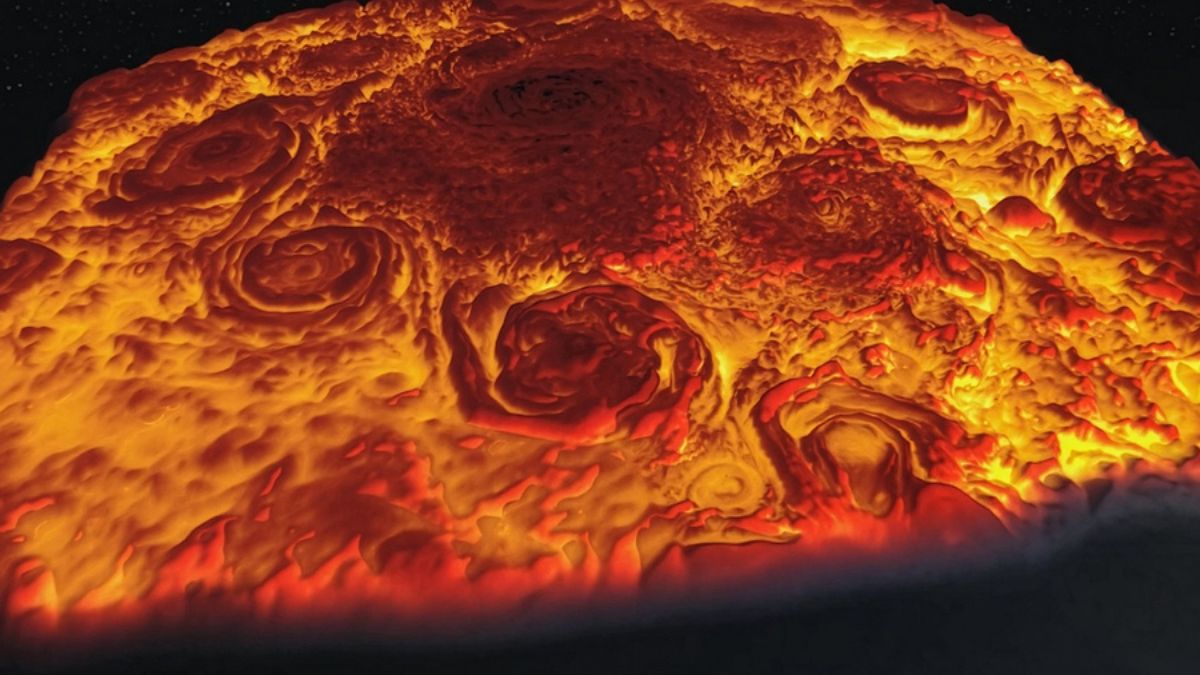
For decades, spacecraft images of Jupiter and Saturn have puzzled planetary scientists. Despite being similar in size and composition, the two gas giants display dramatically different weather systems at their poles. Jupiter hosts a striking formation: a central polar vortex encircled by eight massive storms, resembling a rotating crown. Saturn, by contrast, is capped by a single enormous cyclone, shaped like a near-perfect hexagon.
Now, researchers at the Massachusetts Institute of Technology believe they have identified a key reason behind this cosmic contrast — and the answer may lie deep beneath the planets’ cloud tops.
In a new study published in the Proceedings of the National Academy of Sciences, the MIT team suggests that the structure of a planet’s interior — specifically, how “soft” or “hard” the base of a vortex is — determines whether polar storms merge into one giant system or remain as multiple smaller vortices.
“Our study shows that, depending on the interior properties and the softness of the bottom of the vortex, this will influence the kind of fluid pattern you observe at the surface,” says study author Wanying Kang, assistant professor in MIT’s Department of Earth, Atmospheric and Planetary Sciences (EAPS) in a media release issued by the institute. “I don’t think anyone’s made this connection between the surface fluid pattern and the interior properties of these planets. One possible scenario could be that Saturn has a harder bottom than Jupiter.”
A long-standing planetary mystery
The contrast has been visible for years thanks to two landmark NASA missions. The Juno spacecraft, which has been orbiting Jupiter since 2016, revealed a dramatic polar arrangement of swirling storms, each roughly 3,000 miles wide — nearly half the diameter of Earth. Cassini, which orbited Saturn for 13 years before its mission ended in 2017, documented the planet’s iconic hexagonal polar vortex, stretching nearly 18,000 miles across.
“People have spent a lot of time deciphering the differences between Jupiter and Saturn,” says Jiaru Shi, the study’s first author and an MIT graduate student. “The planets are about the same size and are both made mostly of hydrogen and helium. It’s unclear why their polar vortices are so different.”
Simulating storms on gas giants
To tackle the question, the researchers turned to computer simulations. They created a two-dimensional model of atmospheric flow designed to mimic how storms might evolve on a rapidly rotating gas giant.
While real planetary vortices are three-dimensional, the team argued that Jupiter’s and Saturn’s fast spin simplifies the physics. “In a fast-rotating system, fluid motion tends to be uniform along the rotating axis,” Kang explains. “So, we were motivated by this idea that we can reduce a 3D dynamical problem to a 2D problem because the fluid pattern does not change in 3D. This makes the problem hundreds of times faster and cheaper to simulate and study.”
The model allowed the scientists to test thousands of possible planetary conditions, varying factors such as rotation rate, internal heating, planet size and — crucially — the density of material beneath the vortices. Each simulation began with random chaotic motion and tracked how storms evolved over time.
The outcomes consistently fell into two categories: either the system developed one dominant polar vortex, like Saturn, or several coexisting vortices, like Jupiter.
The decisive factor turned out to be how much a vortex could grow before being constrained by the properties of the layers beneath it.
When the lower layers were made of softer, lighter material, individual vortices could not expand indefinitely. Instead, they stabilized at smaller sizes, allowing multiple storms to coexist at the pole. This matches what scientists observe on Jupiter.
But when the simulated vortex base was denser and more rigid, vortices were able to grow larger and eventually merge. The end result was a single, planet-scale storm — remarkably similar to Saturn’s massive polar cyclone.
“This equation has been used in many contexts, including to model midlatitude cyclones on Earth,” Kang says. “We adapted the equation to the polar regions of Jupiter and Saturn.”
The findings suggest that Saturn’s interior may contain heavier elements or more condensed material than Jupiter’s, giving its atmospheric vortices a firmer foundation to build upon.
“What we see from the surface, the fluid pattern on Jupiter and Saturn, may tell us something about the interior, like how soft the bottom is,” Shi says. “And that is important because maybe beneath Saturn’s surface, the interior is more metal-enriched and has more condensable material which allows it to provide stronger stratification than Jupiter. This would add to our understanding of these gas giants.”
Reading the interiors from the skies
Planetary scientists have long struggled to infer the internal structures of gas giants, where pressures and temperatures are far beyond what can be reproduced in laboratories. This new work offers a rare bridge between visible atmospheric patterns and hidden planetary composition.
Beyond explaining two of the Solar System’s most visually striking storms, the research could shape how scientists interpret observations of distant exoplanets as well — worlds where atmospheric patterns might be the only clues to what lies within.
For now, Jupiter’s swirling crown of storms and Saturn’s solitary hexagon may be doing more than decorating the poles of two distant giants. They may be quietly revealing the deep, unseen architecture of the planets themselves.
Space & Physics
When Quantum Rules Break: How Magnetism and Superconductivity May Finally Coexist
A new theoretical breakthrough from MIT suggests that exotic quantum particles known as anyons could reconcile a long-standing paradox in physics, opening a path to an entirely new form of superconductivity.
For decades, physicists believed that superconductivity and magnetism were fundamentally incompatible. Superconductivity is fragile: even a weak magnetic field can disrupt the delicate pairing of electrons that allows electrical current to flow without resistance. Magnetism, by its very nature, should destroy superconductivity.
And yet, in the past year, two independent experiments upended this assumption.
In two different quantum materials, researchers observed something that should not have existed at all: superconductivity and magnetism appearing side by side. One experiment involved rhombohedral graphene, while another focused on the layered crystal molybdenum ditelluride (MoTe₂). The findings stunned the condensed-matter physics community and reopened a fundamental question—how is this even possible?
Now, a new theoretical study from physicists at the Massachusetts Institute of Technology offers a compelling explanation. Writing in the Proceedings of the National Academy of Sciences, the researchers propose that under the right conditions, electrons in certain magnetic materials can split into fractional quasiparticles known as anyons—and that these anyons, rather than electrons, may be responsible for superconductivity.
If confirmed, the work would introduce a completely new form of superconductivity, one that survives magnetism and is driven by exotic quantum particles instead of ordinary electrons.
“Many more experiments are needed before one can declare victory,” said Senthil Todadri, William and Emma Rogers Professor of Physics at MIT, in a media statement. “But this theory is very promising and shows that there can be new ways in which the phenomenon of superconductivity can arise.”
A Quantum Contradiction Comes Alive
Superconductivity and magnetism are collective quantum states born from the behavior of electrons. In magnets, electrons align their spins, producing a macroscopic magnetic field. In superconductors, electrons pair up into so-called Cooper pairs, allowing current to flow without energy loss.
For decades, textbooks taught that the two states repel each other. But earlier this year, that belief cracked.
At MIT, physicist Long Ju and colleagues reported superconductivity coexisting with magnetism in rhombohedral graphene—four to five stacked graphene layers arranged in a specific crystal structure.
“It was electrifying,” Todadri recalled in a media statement. “It set the place alive. And it introduced more questions as to how this could be possible.”
Soon after, another team reported a similar duality in MoTe₂. Crucially, MoTe₂ also exhibits an exotic quantum phenomenon known as the fractional quantum anomalous Hall (FQAH) effect, in which electrons behave as if they split into fractions of themselves.
Those fractional entities are anyons.
Meet the Anyons: Where “Anything Goes”
Anyons occupy a strange middle ground in the quantum world. Unlike bosons, which happily clump together, or fermions, which avoid one another, anyons follow their own rules—and exist only in two-dimensional systems.
First predicted in the 1980s and named by MIT physicist Frank Wilczek, anyons earned their name as a playful nod to their unconventional behavior: anything goes.
Decades ago, theorists speculated that anyons might be able to superconduct in magnetic environments. But because superconductivity and magnetism were believed to be mutually exclusive, the idea was largely abandoned.
The recent MoTe₂ experiments changed that calculus.
“People knew that magnetism was usually needed to get anyons to superconduct,” Todadri said in a media statement. “But superconductivity and magnetism typically do not occur together. So then they discarded the idea.”
Now, Todadri and MIT graduate student Zhengyan Darius Shi, co-author of the study, revisited the old theory—armed with new experimental clues.
Using quantum field theory, the team modeled how electrons fractionalize in MoTe₂ under FQAH conditions. Their calculations revealed that electrons can split into anyons carrying either one-third or two-thirds of an electron’s charge.
That distinction turned out to be critical.
Anyons are notoriously “frustrated” particles—quantum effects prevent them from moving freely together.
“When you have anyons in the system, what happens is each anyon may try to move, but it’s frustrated by the presence of other anyons,” Todadri explained in a media statement. “This frustration happens even if the anyons are extremely far away from each other.”
But when the system is dominated by two-thirds-charge anyons, the frustration breaks down. Under these conditions, the anyons begin to move collectively—forming a supercurrent without resistance.
“These anyons break out of their frustration and can move without friction,” Todadri said. “The amazing thing is, this is an entirely different mechanism by which a superconductor can form.”
The team also predicts a distinctive experimental signature: swirling supercurrents that spontaneously emerge in random regions of the material—unlike anything seen in conventional superconductors.
Why This Matters Beyond Physics
If experiments confirm superconducting anyons, the implications could extend far beyond fundamental physics.
Because anyons are inherently robust against environmental disturbances, they are considered prime candidates for building stable quantum bits, or qubits—the foundation of future quantum computers.
“These theoretical ideas, if they pan out, could make this dream one tiny step within reach,” Todadri said.
More broadly, the work hints at an entirely new category of matter.
“If our anyon-based explanation is what is happening in MoTe₂, it opens the door to the study of a new kind of quantum matter which may be called ‘anyonic quantum matter,’” Todadri said. “This will be a new chapter in quantum physics.”
For now, the theory awaits experimental confirmation. But one thing is already clear: a rule long thought unbreakable in quantum physics may no longer hold—and the quantum world just became a little stranger, and far more exciting.
-

 Society1 month ago
Society1 month agoThe Ten-Rupee Doctor Who Sparked a Health Revolution in Kerala’s Tribal Highlands
-

 COP303 months ago
COP303 months agoBrazil Cuts Emissions by 17% in 2024—Biggest Drop in 16 Years, Yet Paris Target Out of Reach
-

 Earth3 months ago
Earth3 months agoData Becomes the New Oil: IEA Says AI Boom Driving Global Power Demand
-

 COP303 months ago
COP303 months agoCorporate Capture: Fossil Fuel Lobbyists at COP30 Hit Record High, Outnumbering Delegates from Climate-Vulnerable Nations
-

 Society2 months ago
Society2 months agoFrom Qubits to Folk Puppetry: India’s Biggest Quantum Science Communication Conclave Wraps Up in Ahmedabad
-

 Women In Science4 months ago
Women In Science4 months agoThe Data Don’t Lie: Women Are Still Missing from Science — But Why?
-

 Space & Physics2 months ago
Space & Physics2 months agoIndian Physicists Win 2025 ICTP Prize for Breakthroughs in Quantum Many-Body Physics
-

 Health3 months ago
Health3 months agoAir Pollution Claimed 1.7 Million Indian Lives and 9.5% of GDP, Finds The Lancet